A) nucleotides
B) sugars
C) amino acids
D) nitrogenous bases
E) deoxyribose
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The structural level of a protein least affected by a disruption in hydrogen bonding is the
A) primary level.
B) secondary level.
C) tertiary level.
D) quaternary level.
E) All structural levels are equally affected.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these molecules is not formed by dehydration reactions?
A) fatty acids
B) disaccharides
C) DNA
D) protein
E) amylose
G) B) and E)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The enzyme amylase can break glycosidic linkages between glucose monomers only if the monomers are the α form.Which of the following molecules could amylase break down?
A) glycogen
B) cellulose
C) chitin
D) glycogen and chitin only
E) glycogen, cellulose, and chitin
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You have just had a breakfast of toast (high-fibre bread) with butter and jam (no added sugar) along with a glass of milk. -Of these two,which one will not be hydrolyzed in your digestive tract?
A) fatty acids
B) cellulose
C) starch
D) glucose
E) fructose
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do phospholipids interact with water molecules?
A) The polar heads avoid water, and the nonpolar tails attract water.
B) Phospholipids do not interact with water because water is polar and lipids are nonpolar.
C) The polar heads interact with water, and the nonpolar tails do not.
D) The hydrocarbon tails form hydrogen bonds with water.
E) Both the polar head and lipid backbone from hydrogen bonds with water.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
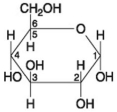 -If two molecules of the general type shown in the above figure were linked together,carbon-1 of one molecule to carbon-4 of the other,the single molecule that would result would be
-If two molecules of the general type shown in the above figure were linked together,carbon-1 of one molecule to carbon-4 of the other,the single molecule that would result would be
A) maltose.
B) fructose.
C) glucose.
D) galactose.
E) sucrose.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You have just had a breakfast of toast (high-fibre bread) with butter and jam (no added sugar) along with a glass of milk. -What are the two major polysaccharides you have consumed?
A) fatty acids and pectin
B) cellulose and fructose
C) starch and fructose
D) glucose and fructose
E) cellulose and starch
G) A) and B)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
The following questions are based on the 15 molecules illustrated in the figure below.Each molecule may be used once,more than once,or not at all.
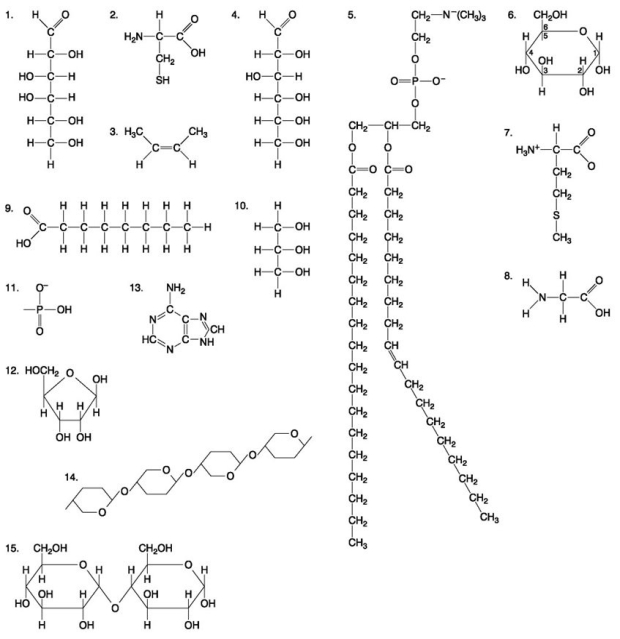 -Which of the following molecules act as building blocks (monomers) of polypeptides?
-Which of the following molecules act as building blocks (monomers) of polypeptides?
A) 1, 4, and 6
B) 2, 7, and 8
C) 7, 8, and 13
D) 11, 12, and 13
E) 12, 13, and 15
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How will brief heating (to 95°C) affect macromolecular structures in aqueous solution?
A) DNA duplexes will unwind and separate.
B) Proteins will unfold (denature) .
C) Starch will hydrolyze into monomeric sugars.
D) Proteins will hydrolyze into amino acids.
E) DNA duplexes will unwind and separate, and proteins will unfold (denature) .
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
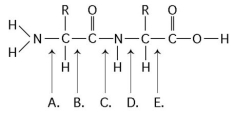 -Which bond is a peptide bond?
-Which bond is a peptide bond?
A) A
B) B
C) C
D) D
E) E
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following are polysaccharides except
A) lactose.
B) glycogen.
C) chitin.
D) cellulose.
E) amylopectin.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The R group or side chain of the amino acid serine is -CH₂-OH.The R group or side chain of the amino acid leucine is -CH₂-CH-(CH₃₎₂.Where would you expect to find these amino acids in a globular protein in aqueous solution?
A) Serine would be in the interior, and leucine would be on the exterior of the globular protein.
B) Leucine would be in the interior, and serine would be on the exterior of the globular protein.
C) Both serine and leucine would be in the interior of the globular protein.
D) Both serine and leucine would be on the exterior of the globular protein.
E) Both serine and leucine would be in the interior and on the exterior of the globular protein.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If cells are grown in a medium containing radioactive ³²P-labelled phosphate,which of these molecules will be labelled?
A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) both phospholipids and nucleic acids
G) A) and C)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Dehydration reactions are used in forming which of the following compounds?
A) triacylglycerides
B) polysaccharides
C) proteins
D) triacylglycerides and proteins only
E) triacylglycerides, polysaccharides, and proteins
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning saturated fats is not true?
A) They are more common in animals than in plants.
B) They have multiple double bonds in the carbon chains of their fatty acids.
C) They generally solidify at room temperature.
D) They contain more hydrogen than unsaturated fats having the same number of carbon atoms.
E) They are one of several factors that contribute to atherosclerosis.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following techniques uses the amino acid sequences of polypeptides to predict a protein's three-dimensional structure?
A) X-ray crystallography
B) bioinformatics
C) analysis of amino acid sequence of small fragments
D) NMR spectroscopy
E) high-speed centrifugation
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular formula for glucose is C₆H₁₂O₆.What would be the molecular formula for a molecule made by linking three glucose molecules together by dehydration reactions?
A) C₁₈H₃₆O₁₈
B) C₁₈H₃₂O₁₆
C) C₆H₁₀O₅
D) C₁₈H₁₀O₁₅
E) C₃H₆O₃
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which modifications of fatty acids will best keep triglycerides solid at warmer temperatures?
A) creating cis double bonds to the fatty acids
B) adding hydrogens to the fatty acids
C) creating trans double bonds to the fatty acids
D) adding hydrogens or trans double bonds to the fatty acids
E) adding cis double bonds and trans double bonds to the fatty acids
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens when nucleotides polymerize to form a nucleic acid?
A) A covalent bond forms between the sugar of one nucleotide and the phosphate of a second nucleotide.
B) A hydrogen bond forms between the sugar of one nucleotide and the phosphate of a second nucleotide.
C) Covalent bonds form between the bases of two nucleotides.
D) Hydrogen bonds form between the bases of two nucleotides.
E) A covalent bond forms between the sugar of one nucleotide and the base of a second nucleotide.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 131
Related Exams